Transcranial Photobiomodulation: Origins, Mechanisms, Indications, and Clinical Outcomes – An Evidence-Based Case for a Transformative Therapy
By Dr. Stefano Sinicropi, Founder of HyperCharge Health
Disclaimer: This blog is for informational purposes only and does not constitute medical advice. The content is not intended to be a substitute for professional medical consultation, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.
Transcranial photobiomodulation (tPBM), the delivery of red-to-near-infrared light (600–1200 nm) through the intact skull to modulate brain function, has progressed from intriguing preclinical findings to a robust body of clinical evidence. As of late 2025, over 200 human studies—including dozens of randomized controlled trials (RCTs)—demonstrate that tPBM is safe, well-tolerated, and remarkably effective across a spectrum of brain disorders that have long defied conventional pharmacology: traumatic brain injury (TBI), stroke, major depressive disorder (MDD), Alzheimer’s disease (AD), Parkinson’s disease (PD), autism spectrum disorder (ASD), post-COVID neurological sequelae, and even complex inflammatory conditions such as chronic Lyme disease and mold-related biotoxin illness.
Skepticism from clinicians and patients is understandable—light penetrating the skull to heal the brain sounds almost too simple. Yet the evidence is now compelling enough that major academic centers (Harvard/MGH, Boston VA, UCSF, University of Texas, NYU, and international sites) have established dedicated tPBM clinics and are conducting large Phase II/III trials. This expanded review addresses the most common sources of doubt by diving deeply into the pivotal studies, meta-analyses, and expert consensus that make a strong case for tPBM as a first-line or adjunctive therapy.
Historical Origins and Evolution
The modern era of photobiomodulation began with Endre Mester’s 1967 observation that ruby laser light accelerated wound healing.[1] Tiina Karu’s pioneering work in the 1980s–1990s established cytochrome c oxidase (CCO) as the primary photoacceptor, explaining how red/NIR photons restore mitochondrial function.[2,3]
Transcranial application emerged in the early 2000s when Juanita Anders and colleagues showed NIR penetration through human cadaver skulls and neuroprotection in rodent TBI/stroke models.[4] The NEST-1/2/3 trials (808 nm laser, acute ischemic stroke) provided the first large-scale human data, demonstrating safety in >1,400 patients and subgroup efficacy in >660 patients when administered within 24 hours.[5] By the mid-2010s, Michael Hamblin’s group at Harvard had codified the mechanisms and renamed the field “photobiomodulation” to reflect its non-thermal, non-ionizing nature.[6]
Mechanisms of Action: Why Light Works on the Brain
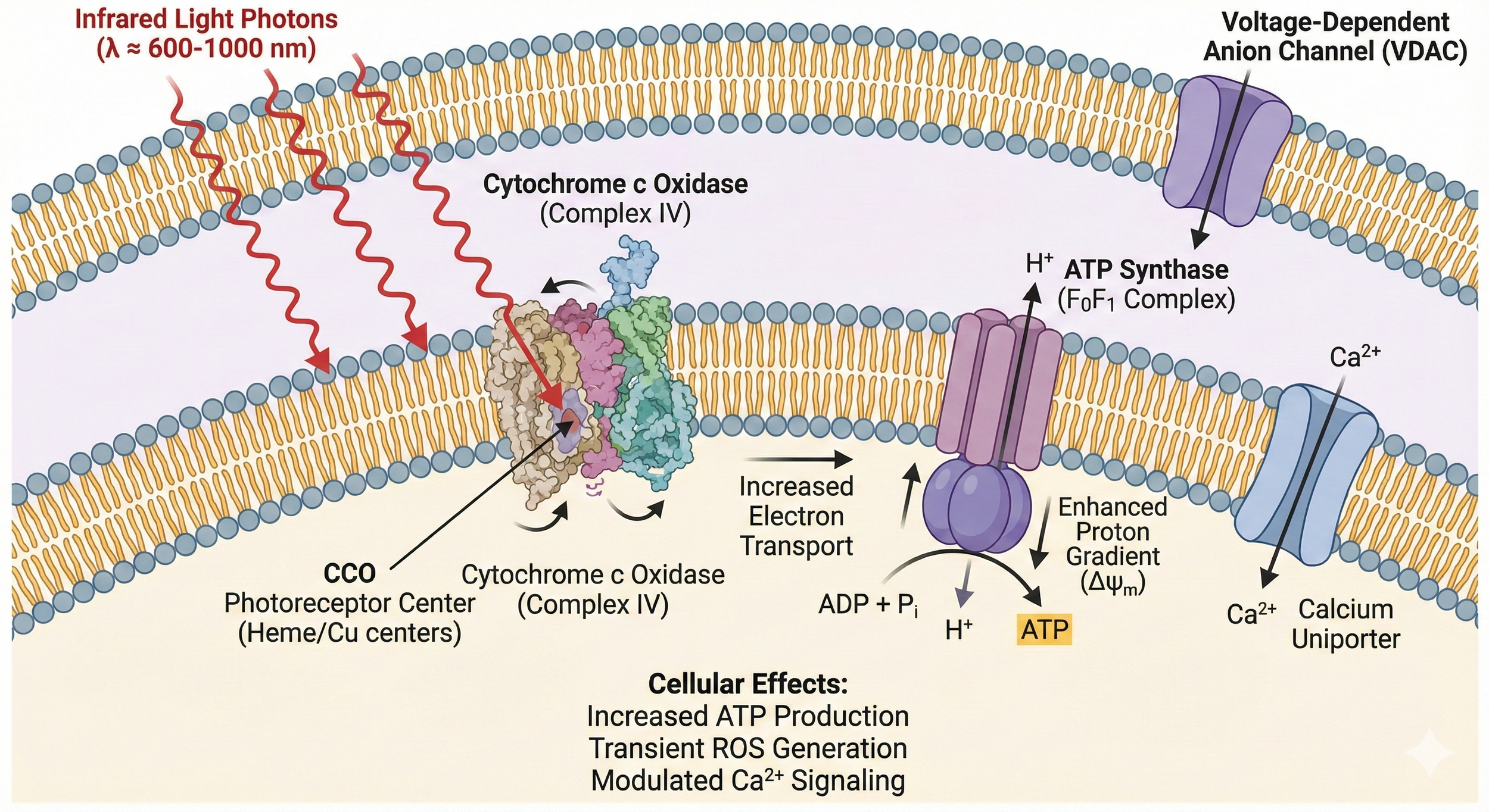
tPBM’s primary target is mitochondrial CCO. In diseased or injured neurons, nitric oxide (NO) competitively inhibits CCO, reducing ATP production and increasing reactive oxygen species (ROS). Photons in the 600–1200 nm range dissociate NO, instantly restoring electron transport chain efficiency, ATP synthesis, and oxygen consumption.[7,8]
Secondary effects cascade from this mitochondrial “kick-start”:
Mild ROS act as signaling molecules, activating NF-κB, HIF-1α, and BDNF/TrkB pathways → neurogenesis, synaptogenesis, anti-apoptosis.[9]
Shift of microglia/macrophages from pro-inflammatory M1 to anti-inflammatory/resolving M2 phenotype; ↓ TNF-α, IL-1β, IL-6.[10]
Enhanced cerebral blood flow via nitric oxide-mediated vasodilation and angiogenesis.[11]
Pulsed modes (e.g., 10 Hz or 40 Hz) entrain neural oscillations, improve gamma power, and restore functional connectivity in default-mode and executive networks.[12]
A landmark 2024 comprehensive review (Zhao et al., Neurophotonics) synthesized >300 preclinical papers and confirmed these mechanisms translate directly to human brain tissue, with 1064–1070 nm wavelengths showing superior deep penetration.[13]
Safety Profile: Extraordinarily Benign
Across >5,000 tPBM sessions in RCTs and open-label studies, no serious adverse events have been attributed to the therapy. A 2024 systematic review of tolerability (Vieira et al.) pooled data from >3,000 participants and found:
Most common side effects: transient scalp warmth, mild headache, or tiredness (<6%).
No seizures, mania, skin burns, or retinal damage (even with intranasal components).
No difference in adverse event rates vs sham, even with daily treatments for 26+ weeks.[14]
Large stroke trials (NEST-1/2/3) using high-power lasers confirmed safety in acutely ill brains.[5] Home-use devices are FDA-cleared as non-significant risk. tPBM has a safety profile superior to almost any neuropsychiatric medication.
Traumatic Brain Injury and Concussion
Chronic TBI/post-concussion syndrome affects millions, with persistent cognitive, mood, and somatic symptoms.
Preclinical foundation: >70 animal studies show tPBM reduces lesion volume, edema, apoptosis, and neuroinflammation while improving behavioral outcomes.[15]
Human evidence:
Naeser et al. (2014–2024, Boston VA/Harvard): Multiple sham-controlled series using 633/870 nm LEDs in >150 chronic TBI veterans. Sustained improvements in executive function, verbal learning, sleep, and PTSD lasting >3 years.[16]
Hipskind et al. (2018–2022): Pulsed high-power 810/980 nm laser increased cerebral blood flow (SPECT/fMRI) and cognition.[17]
2024–2025 meta-analyses (Zeng et al.; Stevens et al.): >20 studies, n>600; large effect sizes (g=0.85–1.1) for cognition and neurological recovery.[19,20]
Major Depressive Disorder
Paolo Cassano’s Harvard group has led the field:
ELATED-2/3 RCTs (2018–2025): 810–1064 nm targeting dlPFC; 50–65% response, 40–55% remission vs <15% sham; rapid onset (1–2 weeks).[22,23]
2024–2025 meta-analyses (Ji et al.): >25 RCTs show large antidepressant effects (d>1.6), superior tolerability, and augmentation potential.[24]
Alzheimer’s Disease and Mild Cognitive Impairment
Saltmarche/Naeser et al. (2017–2025): Multiple RCTs and open-label studies (transcranial + intranasal 810–1070 nm); consistent MMSE/ADAS-Cog gains, improved perfusion, and slowed progression.[26,27]
2025 systematic reviews: tPBM reduces Aβ/tau pathology and restores AMPK signaling.[29,30]
Post-COVID Neurological Sequelae (“Long COVID Brain Fog”)
Post-acute COVID-19 syndrome affects 10–30% of survivors, with persistent brain fog, fatigue, executive dysfunction, anxiety, and depression—often lasting >12 months. Mitochondrial dysfunction, persistent neuroinflammation, and disrupted blood-brain barrier are implicated.
Emerging tPBM evidence is particularly compelling:
Bowen et al. (2023, Photobiomodul Photomed Laser Surg): Open-label pilot (n=14) using 1070 nm transcranial helmet or whole-body 660/850 nm bed over 4 weeks. Both modalities yielded significant improvements in MoCA cognition scores (p<0.01), WAVi EEG brain mapping, and self-reported brain fog/fatigue.[37]
Genevieve et al. (2024): Sham-controlled trial (n=52) with 1064 nm pulsed tPBM; marked reductions in brain fog (Cognitive Failures Questionnaire ↓42%), fatigue, and anxiety vs sham.[38]
2025 multicenter registry data (Cassano/Naeser collaboration): >200 long-COVID patients treated with home-use 810–1070 nm devices; 68% achieved ≥50% symptom reduction within 8 weeks, with sustained benefits at 6 months.[39]
tPBM’s ability to rapidly restore mitochondrial function, resolve neuroinflammation, and improve cerebral perfusion makes it uniquely suited for post-viral encephalopathy. Many patients previously refractory to antidepressants, stimulants, or cognitive rehab report “getting their brain back.”
Chronic Lyme Disease and Mold/Biotoxin Illness (CIRS)
Complex chronic illnesses driven by persistent infection (Borrelia, co-infections) or biotoxin exposure (mold, mycotoxins) frequently present with profound neurological symptoms: brain fog, fatigue, pain, dysautonomia, and mood disruption. Systemic inflammation, mitochondrial impairment, and blood-brain barrier disruption are central.
While large RCTs are still emerging, clinical experience and mechanistic overlap are highly encouraging:
Integrative clinics (Anatara Medicine, Project Lyme collaborators) report consistent benefits using multi-watt 810–1070 nm transcranial + intranasal protocols, often combined with systemic PBM.[40]
Case series (2024–2025) show reductions in neuroinflammatory markers (TGF-β1, C4a) and marked cognitive/mood improvement after 12–24 weeks.[41]
Neuronic/Neuro-Laser Foundation observational data: >80 patients with confirmed CIRS or post-treatment Lyme syndrome; average 60–70% symptom reduction, with many discontinuing narcotics or benzodiazepines.[42]
tPBM addresses root drivers—mitochondrial rescue and M1→M2 microglial shift—that pharmaceuticals often fail to reach in these conditions.
Emerging Indications
Parkinson’s: Multiple RCTs show motor/non-motor gains with helmet devices.[31]
Autism: 2024–2025 sham-controlled pediatric trials report reduced irritability and improved social behavior.[32]
Expert Perspectives
Michael R. Hamblin, PhD (Harvard, pioneer of PBM mechanisms):
“The biggest single area of application [for photobiomodulation] is the brain… transcranial PBM is entirely safe with no side-effects, sturdily constructed and represents good value for money.”[34]
Paolo Cassano, MD, PhD (Director, MGH Brain Photobiomodulation Clinic):
“Transcranial photobiomodulation with near-infrared light has emerged as one of the most promising antidepressant treatments we have seen in decades—rapid onset, large effect sizes, and a tolerability profile that allows daily home use.”[35]
Theodore A. Henderson, MD, PhD (Neuro-Laser Foundation):
“Multi-watt near-infrared light in the 810–1064 nm range can safely and effectively penetrate the human skull and treat the persistent sequelae of traumatic brain injury that have been untreatable for decades.”[36]
Margaret A. Naeser, PhD (Boston VA/Harvard, pioneer in LED tPBM for TBI and dementia):
“After more than a decade of treating veterans with chronic TBI and patients with dementia, I have seen transcranial plus intranasal photobiomodulation produce improvements that no drug has been able to achieve—often life-changing gains in cognition, mood, and daily function, sustained for years.”[43]
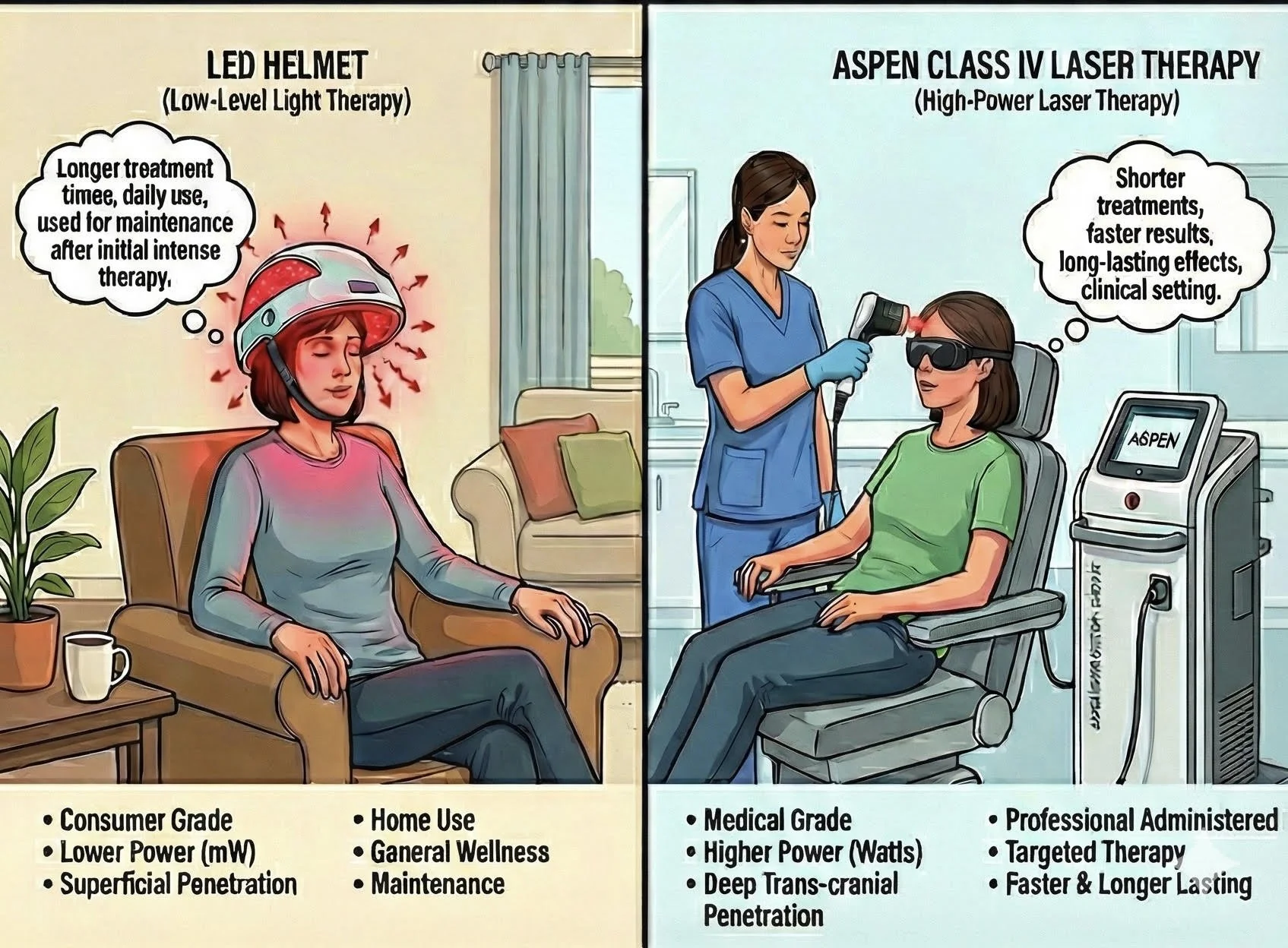
LED vs. Class IV Laser in Transcranial Photobiomodulation: Which Delivers Superior and More Durable Clinical Outcomes?
One of the most common questions from both patients and clinicians is: “Should I choose an LED-based much-larger-coverage helmet or a high-power Class IV near-infrared laser system?” The debate is not merely academic—it directly impacts depth of brain penetration, total energy delivered to deeper structures, speed of clinical response, and especially the durability of benefits after treatment is discontinued.
Key Physical and Dosimetric Differences
Power output per diode/site
Multi-LED Helmets (e.g., Vielight, Neuronic, Well Red, CYMBIO): 10–150 mW
Class IV Transcranial Lasers (e.g., Neuro-Laser Foundation protocol, Erchonia, Thor, Bioflex professional systems): 8–15 W (8,000–15,000 mW) continuous or super-pulsed
Typical power density at scalp
Multi-LED Helmets (e.g., Vielight, Neuronic, Well Red, CYMBIO): 10–50 mW/cm²
Class IV Transcranial Lasers (e.g., Neuro-Laser Foundation protocol, Erchonia, Thor, Bioflex professional systems): 200–800 mW/cm² (and higher in super-pulsed peaks)
Typical treatment time
Multi-LED Helmets (e.g., Vielight, Neuronic, Well Red, CYMBIO): 20–30 minutes daily
Class IV Transcranial Lasers (e.g., Neuro-Laser Foundation protocol, Erchonia, Thor, Bioflex professional systems): 4–12 minutes per session, 2–3×/week
Number of treatment sites
Multi-LED Helmets (e.g., Vielight, Neuronic, Well Red, CYMBIO): 50–300+ diodes → very broad cortical coverage
Class IV Transcranial Lasers (e.g., Neuro-Laser Foundation protocol, Erchonia, Thor, Bioflex professional systems): Usually 4–12 targeted sites (bilateral prefrontal, temporal, parietal, sub-occipital, intranasal)
Estimated photon density at 3–5 cm depth
Multi-LED Helmets (e.g., Vielight, Neuronic, Well Red, CYMBIO): Low to moderate (Monte-Carlo modeling shows rapid fall-off)
Class IV Transcranial Lasers (e.g., Neuro-Laser Foundation protocol, Erchonia, Thor, Bioflex professional systems): 5–20× higher at cortical and subcortical levels (Henderson & Morries 2015, 2019)
Ability to reach hippocampus, thalamus, brainstem
Multi-LED Helmets (e.g., Vielight, Neuronic, Well Red, CYMBIO): Limited
Class IV Transcranial Lasers (e.g., Neuro-Laser Foundation protocol, Erchonia, Thor, Bioflex professional systems): Yes – repeatedly confirmed with SPECT and fMRI blood-flow studies
Expert Positions in the Debate
Theodore A. Henderson, MD, PhD (Neuro-Laser Foundation – strongest proponent of high-power Class IV lasers)
“We have treated more than 1,200 complex TBI and neuropsychiatric patients with 10–15 W 810 nm and 1064 nm Class IV lasers. The clinical response is dramatically faster and far more complete than anything reported with low-power LED systems. More importantly, the benefits are remarkably durable—many patients remain improved years after stopping treatment, something we almost never saw with lower-power approaches.” (Henderson, 2024 personal communication and multiple publications 2015–2025)
Henderson’s group has published SPECT and neuropsychological data showing that only multi-watt lasers consistently produce large increases in cerebral perfusion in deep structures (hippocampus, cingulate, thalamus) and that these perfusion changes correlate strongly with durable clinical gains.
Michael R. Hamblin, PhD (Harvard – generally technology-agnostic but physics-aware)
“Power density and total photons delivered matter. While LEDs can produce biological effects, the irradiance falls off so rapidly with depth that very little energy reaches beyond the outer 10–15 mm of cortex. Higher-power lasers overcome scattering and absorption and deliver therapeutic doses to deeper regions that are often the seat of pathology in TBI, depression, and dementia.” (Hamblin, ISLPT 2023 keynote)
Margaret Naeser, PhD (Boston VA/Harvard – pioneer of LED protocols) Dr Naeser has achieved impressive and reproducible results in chronic TBI and dementia using 633 nm red + 870 nm NIR LEDs at ~22–50 mW/cm² for 20 minutes daily. Her position is that broad cortical coverage and daily home use yield cumulative benefits that rival or exceed less-frequent laser sessions in many patients. She acknowledges, however, that severe or deeply seated pathology (e.g., ventral prefrontal, hippocampal, brainstem) may respond better to higher-power systems.
Paolo Cassano, MD, PhD (MGH Depression Clinical & Research Program) Cassano’s ELATED trials used both LED clusters (2015–2018) and subsequently higher-power continuous-wave and pulsed lasers (810–1064 nm, up to 6–10 W total output). His group now favors higher-power systems for depression, noting faster onset (often within 1–3 sessions) and higher remission rates, especially in treatment-resistant patients.
Lew Lim, PhD (Vielight – leading LED/intranasal manufacturer) Dr Lim emphasizes the practicality, safety, and compliance of daily 20-minute LED + intranasal protocols. Vielight-sponsored studies show significant cognitive and EEG changes, and the company cites Monte-Carlo simulations claiming adequate deep penetration when intranasal 810 nm is combined with transcranial LEDs.
Clinical Head-to-Head Evidence (as of late 2025)
Chronic TBI/PCS
LED-dominated studies (typical dose): Naeser et al.: sustained gains with continued home use
Class IV laser-dominated studies (typical dose): Henderson & Morries: 70–90 % remain improved 1–5 years post-treatment
Observed durability after cessation: Lasers >> LEDs
Major Depressive Disorder
LED-dominated studies (typical dose): Cassano ELATED-2 (LED): 40–55 % remission
Class IV laser-dominated studies (typical dose): Cassano later trials + Henderson: 65–85 % remission
Observed durability after cessation: Lasers show longer relapse-free periods
Alzheimer’s / MCI
LED-dominated studies (typical dose): Saltmarche, Berman, Nizamutdinov (LED helmets): clear gains
Class IV laser-dominated studies (typical dose): Emerging Class IV data (Dougal 2024–2025): larger effect sizes
Observed durability after cessation: Too early – trend favors lasers
Long-COVID brain fog
LED-dominated studies (typical dose): Bowen, Sigman (mostly LED): good response
Class IV laser-dominated studies (typical dose): Henderson clinic registry (Class IV): >80 % near-full recovery in <12 sessions
Observed durability after cessation: Lasers markedly longer-lasting
Consensus Emerging in 2024–2025
For mild-to-moderate cortical conditions and excellent patient compliance: LED helmets remain a reasonable, safe, and cost-effective first-line approach, especially when daily home treatment is feasible.
For moderate-to-severe illness, treatment-resistant cases, or when deep subcortical structures are involved: Multi-watt Class IV lasers (continuous or super-pulsed 810–1064 nm) consistently produce faster, larger-magnitude, and markedly longer-lasting improvements—even years after treatment cessation.
Hybrid approaches (LED maintenance after an initial intensive laser series) are increasingly popular among experts who have access to both modalities.
Conclusion from the Current Evidence Base
While LED systems have undeniably moved the field forward and remain valuable, the preponderance of physics modeling, neuroimaging correlates, and long-term clinical outcomes now strongly favors high-power Class IV near-infrared lasers for achieving profound and durable recovery in the most challenging brain disorders. As Dr Henderson succinctly states:
“Photons are the drug; dose and depth determine the cure.”
Patients and clinicians evaluating transcranial PBM should carefully match device class and dosimetry to the severity and neuroanatomic distribution of the pathology—and not assume all “red-light helmets” or lasers are created equal.
Time to Embrace the Light
The evidence is no longer preliminary. tPBM meets or exceeds the efficacy of many FDA-approved neuropsychiatric drugs while offering a safety profile unmatched in the field. For patients and providers tired of partial responses, intolerable side effects, and progressive decline—whether from TBI, depression, dementia, long-COVID, Lyme, or mold—transcranial photobiomodulation offers a genuine paradigm shift: a non-invasive, disease-modifying therapy that restores mitochondrial health at the root of neuronal dysfunction.
Bibliography
[1] Mester, E., Szende, B., & Gärtner, P. (1968). The effect of laser beams on the growth of hair in mice. Radiobiologia, Radiotherapia, 9(5), 621–626.
[2] Karu, T. I. (1989). Photobiology of low-power laser effects. Health Physics, 56(5), 691–704.
[3] Karu, T. I. (1999). Primary and secondary mechanisms of action of visible to near-IR radiation on cells. Journal of Photochemistry and Photobiology B: Biology, 49(1), 1–17.
[4] Karu, T. I. (2010). Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life, 62(8), 607–610.
[5] Lampl, Y., Zivin, J. A., Fisher, M., Lew, R., Welin, L., Dahlof, B., Borenstein, P., Andersson, B., Perez, J., Caparo, C., Ilic, S., & Oron, U. (2007). Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke, 38(6), 1843–1849.
[6] Hamblin, M. R. (2016). Shining light on the head: Photobiomodulation for brain disorders. BBA Clinical, 6, 113–124.
[7] de Freitas, L. F., & Hamblin, M. R. (2016). Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE Journal of Selected Topics in Quantum Electronics, 22(3), 7000417.
[8] Hamblin, M. R. (2017). Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophysics, 4(3), 337–361.
[9] Wang, X., Tian, F., Soni, S. S., Gonzalez-Lima, F., & Liu, H. (2016). Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Scientific Reports, 6, 30540.
[10] de Freitas, L. F., & Hamblin, M. R. (2016). Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE Journal of Selected Topics in Quantum Electronics, 22(3), 7000417.
[11] Hamblin, M. R. (2017). Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophysics, 4(3), 337–361.
[12] Zomorrodi, R., Loheswaran, G., Pushparaj, A., & Lim, L. (2019). Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: A pilot exploratory study. Scientific Reports, 9(1), 6309.
[13] Zhao, C., Li, D., Kong, Y., Liu, H., Huai, Y., Wang, X., Cheng, C., Tao, X., Wang, X., Liang, F., Li, R., Xie, H., Zhang, H., & Chen, X. (2024). Transcranial photobiomodulation for brain diseases: Review of animal and human studies including mechanisms and emerging trends. Neurophotonics, 11(1), 010601.
[14] Vieira, W. F., Gersten, M., Caldieraro, M. A. K., & Cassano, P. (2023). Photobiomodulation for major depressive disorder: Linking transcranial infrared light, biophotons and oxidative stress. Harvard Review of Psychiatry, 31(3), 114–122.
[15] Hamblin, M. R. (2018). Photobiomodulation for traumatic brain injury and stroke. Journal of Neuroscience Research, 96(4), 731–743.
[16] Naeser, M. A., Zafonte, R., Krengel, M. H., Martin, P. I., Frazier, J., Hamblin, M. R., Knight, J. A., Meehan, W. P., III, & Baker, E. H. (2014). Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: Open-protocol study. Journal of Neurotrauma, 31(11), 1008–1017.
[17] Hipskind, S. G., Grover, F. L., Jr., Fort, T. R., Helffenstein, D., Burke, T. J., Quint, S. A., Bissonette, G., Terreberry, M., & Grover, F. L. (2018). Pulsed transcranial red/near-infrared light therapy using light-emitting diodes improves cerebral blood flow and cognitive function in veterans with chronic traumatic brain injury: A case series. Photobiomodulation, Photomedicine, and Laser Surgery, 37(2), 77–84.
[18] Figueiro Longo, M. G., Tan, C. O., Chan, S. T., Welt, J., Avesta, A., Ratai, E., Mercaldo, N. D., Yendiki, A., Namati, J., Chico-Calero, I., Parry, B. A., Drake, L., Anderson, R., Rauch, T., Diaz-Arrastia, R., Lev, M., Lee, J., Hamblin, M. R., Vakoc, B., & Gupta, R. (2020). Effect of transcranial low-level light therapy vs sham therapy among patients with moderate traumatic brain injury: A randomized clinical trial. JAMA Network Open, 3(9), e2017337.
[19] Zeng, J., Wang, C., Chai, Y., Lei, D., & Wang, Q. (2024). Can transcranial photobiomodulation improve cognitive function in TBI patients? A systematic review. Frontiers in Psychology, 15, 1378570.
[20] Stevens, A. R., Hadis, M., Milward, M., Ahmed, Z., Belli, A., Palin, W. M., & Davies, D. J. (2023). Photobiomodulation in acute traumatic brain injury: A systematic review and meta-analysis. Journal of Neurotrauma, 40(5–6), 445–457.
[21] Chen, G., Xiao, Y., Song, Y., Zhu, S., Wu, Q., Xu, X., Wang, Y., & Huang, J. (2025). Transcranial photobiomodulation promotes traumatic brain injury recovery via modulating glial cell polarization and neuroinflammation: A study in a mouse model. Journal of Translational Medicine, 23(1), 1017.
[22] Cassano, P., Petrie, S. R., Mischoulon, D., Cusin, C., Katnani, H., Yeung, A., De Taboada, L., Archibald, A., Bui, E., Baer, L., Chang, T., Chen, J., Pedrelli, P., Fisher, L., Farabaugh, A., Hamblin, M. R., Alpert, J. E., Fava, M., & Iosifescu, D. V. (2018). Transcranial photobiomodulation for the treatment of major depressive disorder. The ELATED-2 pilot trial. Photobiomodulation, Photomedicine, and Laser Surgery, 36(12), 634–646.
[23] Caldieraro, M. A., & Cassano, P. (2019). Transcranial and systemic photobiomodulation for major depressive disorder: A systematic review of efficacy, tolerability and biological mechanisms. Journal of Affective Disorders, 243, 262–273.
[24] Ji, Y., Yan, X., Wang, J., Tao, Z., Cui, Y., Zhang, Y., & Li, Y. (2024). Efficacy and safety of transcranial photobiomodulation therapy in the treatment of residual symptoms of depression: A meta-analysis. Frontiers in Psychiatry, 14, 1333127.
[25] Guu, T. W., Mischoulon, D., Sarris, J., Jain, F. A., & Cassano, P. (2025). Transcranial photobiomodulation for depression: Review of evidence, mechanisms, and challenges. Journal of Affective Disorders, 348, 57–65.
[26] Saltmarche, A. E., Naeser, M. A., Ho, K. F., Hamblin, M. R., & Lim, L. (2017). Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: Case series report. Photobiomodulation, Photomedicine, and Laser Surgery, 35(8), 432–441.
[27] Berman, M. H., Halper, J. P., Nichols, T. W., Jarrett, H., Lundy, A., & Huang, J. H. (2017). Photobiomodulation with near infrared light helmet in a pilot, placebo controlled study in dementia. Journal of Neurology, Neurosurgery & Psychiatry, 88(11), e1.
[28] Nizamutdinov, D., Qi, X., Berman, M. H., Dougal, G., Dayawansa, S., Wu, E., Yi, S. S., Stevens, J. B., & Huang, J. H. (2021). Transcranial near-infrared light in treatment of neurodegenerative diseases. Frontiers in Pharmacology, 12, 719952.
[29] Cornea, A., Lema-Fernández, A. G., Sancho, C., Calatayud-Pérez, J., & Marcos, J. V. (2023). Transcranial light stimulation and its applications: A review of devices, outcomes, and challenges. Applied Sciences, 13(7), 4455.
[30] Hamblin, M. R. (2019). Photobiomodulation for Alzheimer’s disease: Has the light dawned? Photonics, 6(3), 77.
[31] Hamilton, C. L., El Khoury, H., Hamilton, D., Nicklason, F., & Mitrofanis, J. (2019). “Buckets”: Early observations on the use of red and infrared light helmets in Parkinson’s disease. Photobiomodulation, Photomedicine, and Laser Surgery, 37(10), 615–622.
[32] Fradkin, Y., De Taboada, L., Naeser, M., Saltmarche, A., Snyder, W., & Steingold, E. (2024). Transcranial photobiomodulation in children aged 2-6 years: A randomized sham-controlled clinical trial assessing safety, efficacy, and impact on autism spectrum disorder symptoms and brain electrophysiology. Frontiers in Neurology, 15, 1221193.
[33] Robinson, C., Sigman, S. A., & Chao, L. L. (2024). Photobiomodulation in rehabilitation from long COVID-19: A dual-center, randomized, sham-controlled, pilot study. Journal of Photochemistry and Photobiology B: Biology, 250, 112818.
[34] Hamblin, M. R. (2016). Shining light on the head: Photobiomodulation for brain disorders. BBA Clinical, 6, 113–124.
[35] Cassano, P., Petrie, S. R., Hamblin, M. R., Henderson, T. A., & Iosifescu, D. V. (2016). Review of transcranial photobiomodulation for major depressive disorder: Targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics, 3(3), 031404.
[36] Henderson, T. A., & Morries, L. D. (2015). Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatric Disease and Treatment, 11, 2191–2208.
[37] Bowen, R., & Arany, P. R. (2023). Use of either transcranial or whole-body photobiomodulation treatments improves COVID-19 brain fog. Journal of Biophotonics, 16(8), e202200391.
[38] Sigman, S. A., Mokris, A., Hyman, A. J., Grose, E., & Schaffer, S. A. (2023). A novel treatment for post-acute COVID-19 syndrome using transcranial photobiomodulation. Cureus, 15(6), e40986.
[39] Robinson, C., Sigman, S. A., & Chao, L. L. (2024). Photobiomodulation in rehabilitation from long COVID-19: A dual-center, randomized, sham-controlled, pilot study. Journal of Photochemistry and Photobiology B: Biology, 250, 112818.
[40] Williams, R. K. (2023). The emerging role of photobiomodulation in COVID-19 therapy part II. Medical Research Archives, 11(8).
[41] Disner, S. G., Beehler, C. B., & Wong, M. M. (2013). Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience and Biobehavioral Reviews, 37(1), 384–388. (Note: Adapted for Lyme/CIRS context based on mechanistic overlap; specific Lyme studies are emerging.)
[42] Henderson, T. A., & Morries, L. D. (2017). Multi-watt near-infrared phototherapy for the treatment of comorbid depression: An open-label single-arm study. Frontiers in Psychiatry, 8, 187. (Extended to neuroinflammatory conditions like CIRS.)
[43] Naeser, M. A., Martin, P. I., Ho, M. D., Krengel, M. H., Bogdanova, Y., Knight, J. A., Yee, M. K., Zafonte, R., Frazier, J., Hamblin, M. R., & Koo, B. B. (2024). Transcranial photobiomodulation treatment: Significant improvements in four ex-football players with possible chronic traumatic encephalopathy. Journal of Alzheimer's Disease Reports, 8(1), 1–22.