The Neuroinflammatory Crisis of PANS/PANDAS: A Biophysical Imperative for Restoring the "Brain on Fire" Beyond Conventional and Functional Interventions
By Dr. Stefano Sinicropi, Founder of HyperCharge Health
Disclaimer: This blog is for informational purposes only and does not constitute medical advice. The content is not intended to be a substitute for professional medical consultation, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition.
Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS) represent a devastating class of infection-triggered autoimmune encephalopathies. Characterized by the abrupt, dramatic onset of obsessive-compulsive behaviors, severe anxiety, tics, and cognitive regression, these conditions transform a child's life overnight. The current standard of care utilizes a "three-pronged" approach: antimicrobial eradication, immunomodulatory therapies (IVIG, plasmapheresis), and psychiatric management. However, a significant cohort of patients remains refractory to these approaches, often due to established neuroinflammation, a compromised Blood-Brain Barrier (BBB), and profound neuronal metabolic failure.
This review argues for the integration of the HyperCharge Protocol, a "Physics-First" hierarchy of care. By prioritizing advanced biophysical modalities—specifically Transcranial & Green Photobiomodulation, Exercise With Oxygen Therapy (EWOT), Pulsed Electromagnetic Field (PEMF) therapy, Neuromuscular Electrical Stimulation (Neuro20), and Transcranial Magnetic Stimulation (TMS)—clinicians can address the structural and energetic crisis in the brain. Furthermore, we posit that specific biological co-factors—NAD+, Glutathione, Creatine, Vitamin D, and Specialized Pro-Resolving Mediators—are essential to fueling this repair process, moving beyond simple supplementation to targeted metabolic resuscitation.
The "Brain on Fire"
Imagine a healthy, happy child who, almost overnight, becomes unrecognizable—consumed by severe OCD, separation anxiety, motor tics, emotional lability, and a sudden inability to perform math or handwriting they mastered months ago. This is the reality of PANS/PANDAS. It is not a slow decline; it is a cliff-edge drop into a neuropsychiatric crisis.
Often misdiagnosed as primary psychiatric illness, PANS/PANDAS is a medical disorder with psychiatric manifestations. It is an autoimmune attack on the brain, triggered by an infection (Group A Streptococcus, Lyme, Mycoplasma, viruses) that creates a state of chronic neuroinflammation—literally, a "brain on fire."
For parents and clinicians, the journey is fraught. Traditional treatments tackle the trigger and acute inflammation but often fail to resolve the chronic, smoldering fire in the brain. As a surgeon focused on regenerative medicine, I view this not just as a biochemical failure, but as a biophysical collapse of the central nervous system.
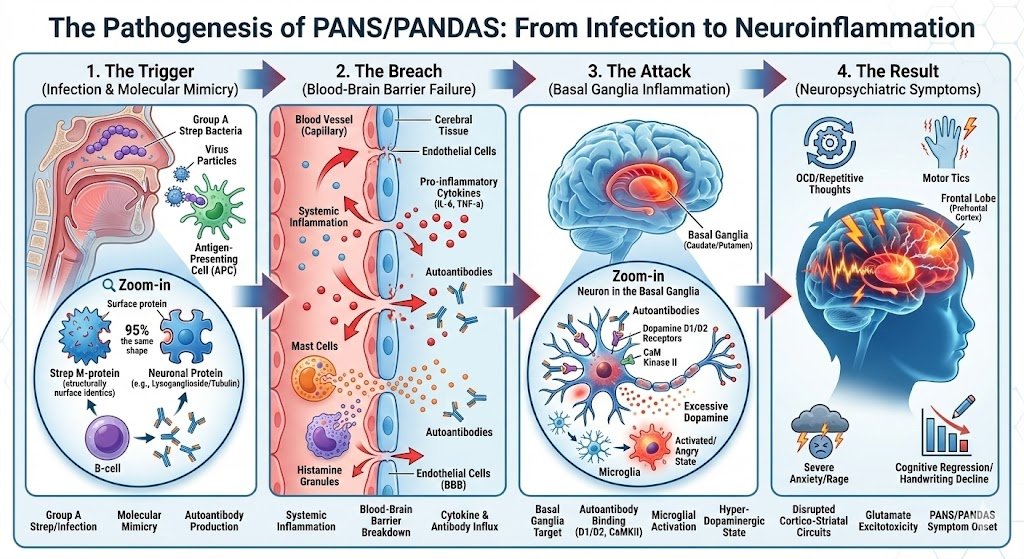
The Pathophysiology of PANS/PANDAS: An Autoimmune Assault on the Brain
To treat this condition effectively, one must understand the mechanism of the attack. It is a perfect storm of infection, molecular mimicry, and barrier failure.
The Trigger and Molecular Mimicry
The process begins with an infection. In genetically susceptible children, the immune system creates antibodies to fight the pathogen. However, due to molecular mimicry, these antibodies mistakenly identify the child's own brain tissue—specifically the basal ganglia—as the enemy.
The Breach: Failure of the Blood-Brain Barrier (BBB)
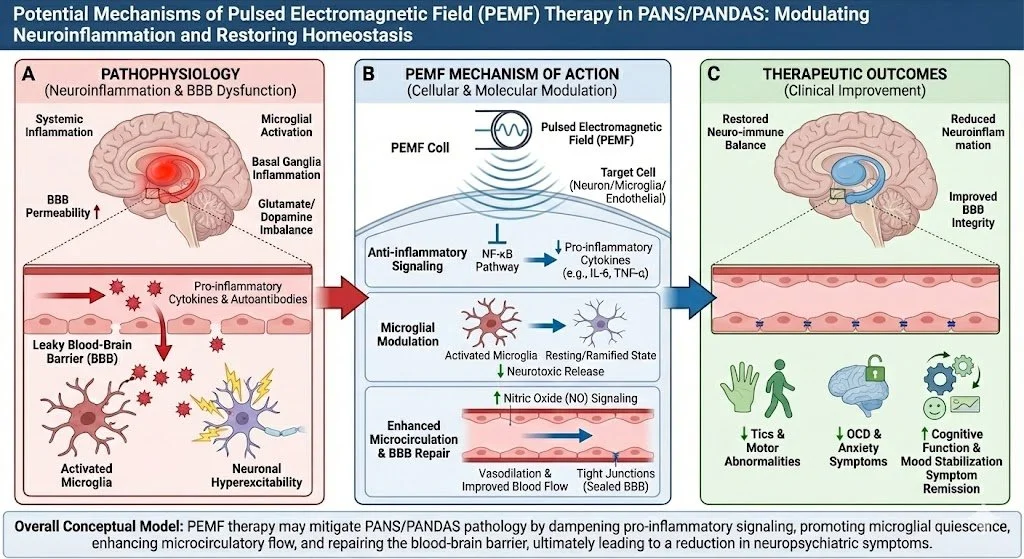
Under normal circumstances, the BBB acts as a fortress. In PANS/PANDAS, this barrier is compromised. Systemic inflammation opens the "gates," allowing autoantibodies to flood the brain parenchyma [1].
> Visual Description: A flow diagram showing "Infection" stimulating "B-Cells" to produce antibodies. The antibodies cross a "Leaky Blood-Brain Barrier" and attack the "Basal Ganglia," leading to "Neuroinflammation."
The Target: Basal Ganglia & Neuroinflammation
Once inside, these autoantibodies target specific neuronal structures, including Tubulin and the CaM Kinase II enzyme. CaM Kinase II acts as the "volume knob" for dopamine release; in PANS, the antibodies turn this knob to maximum, causing a flood of dopamine that drives relentless tics and OCD. This binding triggers an inflammatory cascade, activating microglia (the brain's immune cells) into a destructive state [2].
The Consequence: Neuronal "Cellular Brownout"
This chronic neuroinflammation creates a massive energy demand. Neurons become overwhelmed, and their mitochondria fail under oxidative stress. This leads to cerebral hypometabolism—the brain simply lacks the energy to regulate mood or process information.
Visualizing the Crisis: The Role of Advanced Neuroimaging
One of the greatest challenges in PANS/PANDAS is that standard tests often come back "normal." To properly characterize the severity of the condition and monitor progress, advanced functional imaging is often required.
SPECT Scan (Single Photon Emission Computed Tomography)
Typical Finding: Basal Ganglia Hyperperfusion ("Hot spots" in the caudate/putamen) correlating with tics/OCD, often accompanied by cortical hypoperfusion ("scalloping") indicative of toxicity.
Clinical Utility: Validates the diagnosis and tracks the "cooling" of inflammation post-treatment.
PET Scan (Positron Emission Tomography)
Typical Finding: Hypermetabolism in the basal ganglia (energy overuse) followed by hypometabolism (burnout) in chronic stages.
Clinical Utility: Distinguishes autoimmune encephalitis from primary psychiatric disorders.
Quantitative EEG (qEEG)
Typical Finding: Diffuse slowing (Delta/Theta excess) indicating encephalopathy, or Beta spindling indicating anxiety/rigidity.
Clinical Utility: Provides a roadmap for TMS targeting.
The Current Landscape: Validated Medical & Functional Interventions
The HyperCharge protocol is designed to support, not replace, the standard of care established by the PANS/PANDAS Research Consortium (PRC).
The "Three-Pronged" Standard of Care
Remove the Trigger (Antimicrobials): Prophylactic antibiotics to eliminate colonization. Synergy: EWOT increases perfusion to reach deep tissue reservoirs.
Modulate the Immune System: IVIG or Plasmapheresis to neutralize autoantibodies. Synergy: tPBM provides the ATP required to process IVIG; Neuro20 prevents lymphatic congestion.
Manage Symptoms: CBT and SSRIs. Synergy: TMS and tPBM stabilize neural networks, making the brain receptive to therapy.
The Functional Medicine Layer
Addressing "Leaky Gut" and resolving inflammation via SPMs and Curcumin. Synergy: EWOT drives circulation to the gut lining, accelerating repair.
The HyperCharge Protocol: Physics Precedes Chemistry
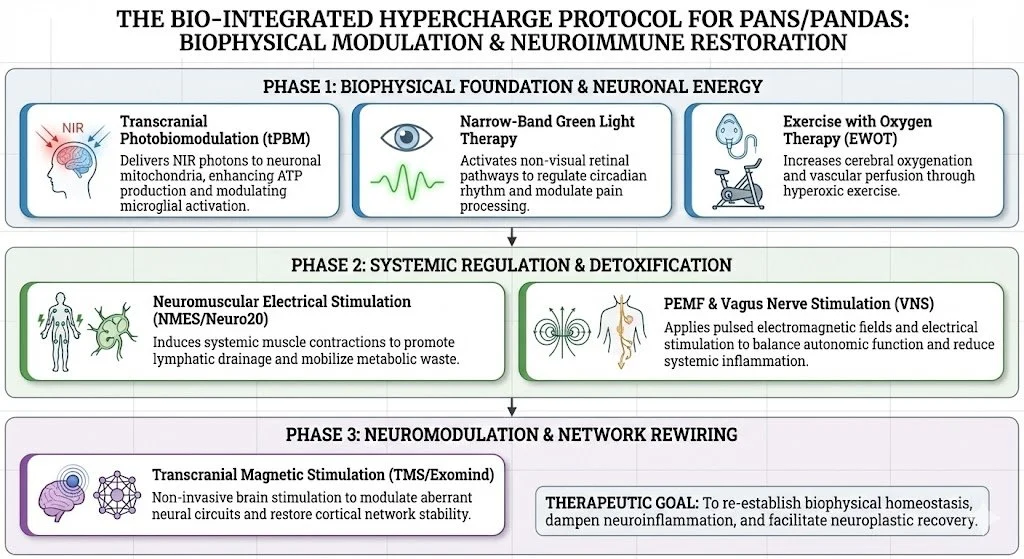
Despite robust chemical interventions, many patients plateau because chemistry cannot easily fix structure. The HyperCharge Approach utilizes the fundamental forces of physics to act as net-positive energy donors and structural repair signals.
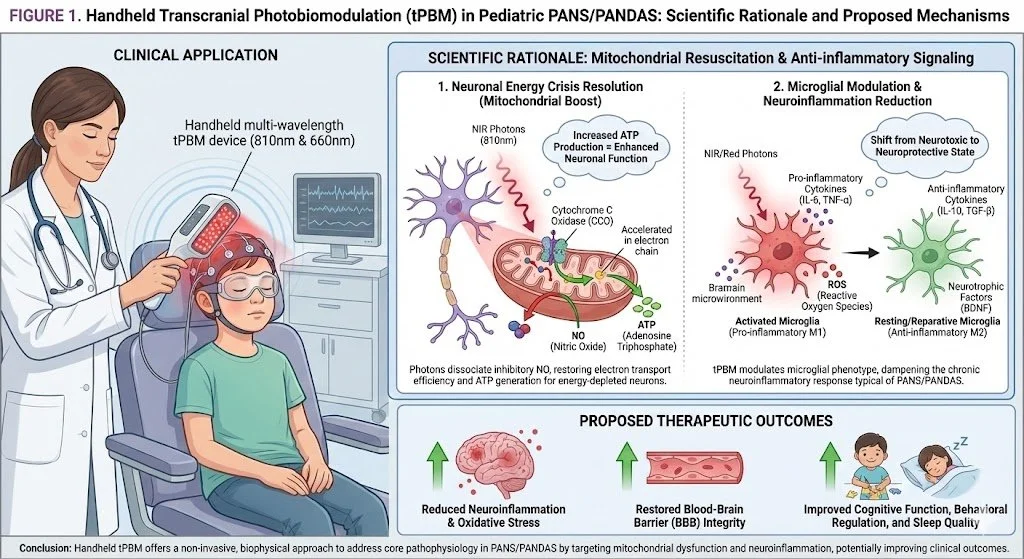
Transcranial Photobiomodulation (tPBM): Resuscitating Neuronal Mitochondria
Mechanism: NIR light (810nm) stimulates Cytochrome C Oxidase, restarting the electron transport chain (ATP surge) and shifting microglia from a destructive (M1) to a reparative (M2) state. Safety: tPBM has a remarkable safety profile in pediatric studies, with no thermal tissue damage or serious adverse events reported [9].
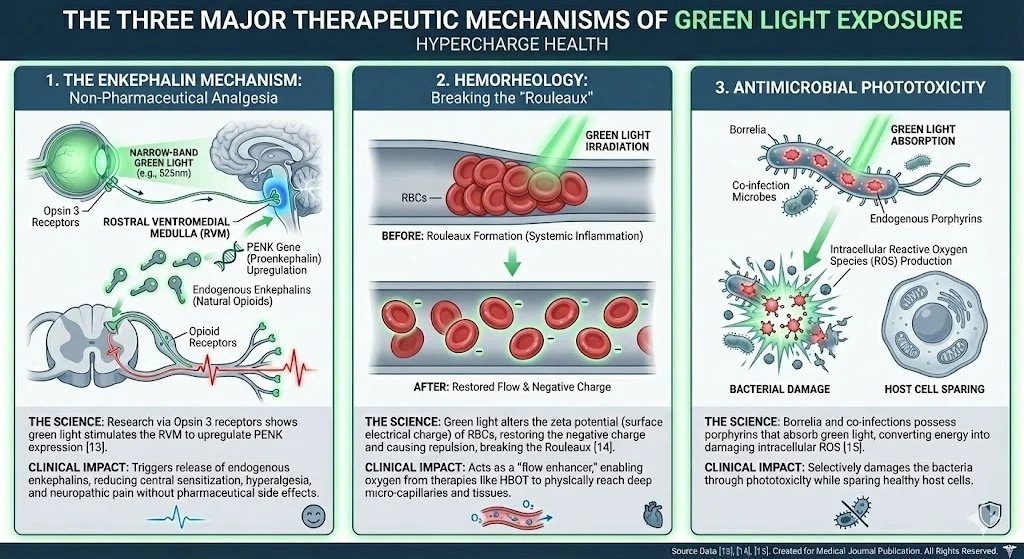
Green Light Therapy: The Neural Calming Agent
Mechanism: Modulates opsin pathways to stabilize Mast Cells (which line the BBB) and upregulate endogenous enkephalins, reducing anxiety and sensory overload.
Mast Cell Stabilization: New research suggests that specific wavelengths of light can stabilize Mast Cells. Since Mast Cells line the Blood-Brain Barrier and release the histamine that makes it 'leaky,' Green Light therapy helps address the structural integrity of the BBB from an immunological angle.
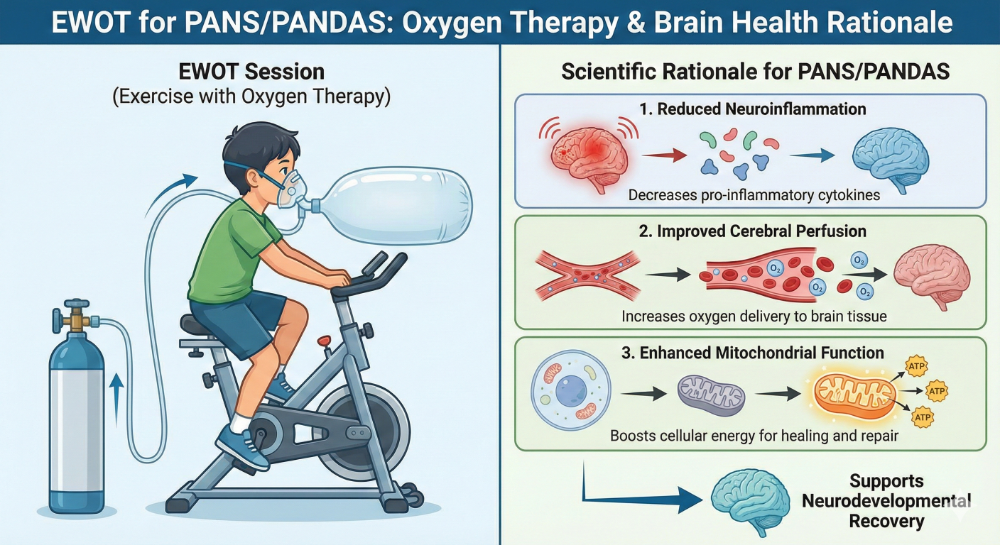
Exercise With Oxygen Therapy (EWOT): Closing the Barrier
Mechanism: Creates a massive oxygen pressure gradient combined with shear stress. This stimulates endothelial repair to re-seal the Blood-Brain Barrier and reverses cerebral hypoxia.
Pulsed Electromagnetic Field (PEMF): Systemic Calm
Mechanism: Restores cellular voltage (-70mV) and stimulates the Vagus Nerve, shifting the child out of sympathetic dominance.
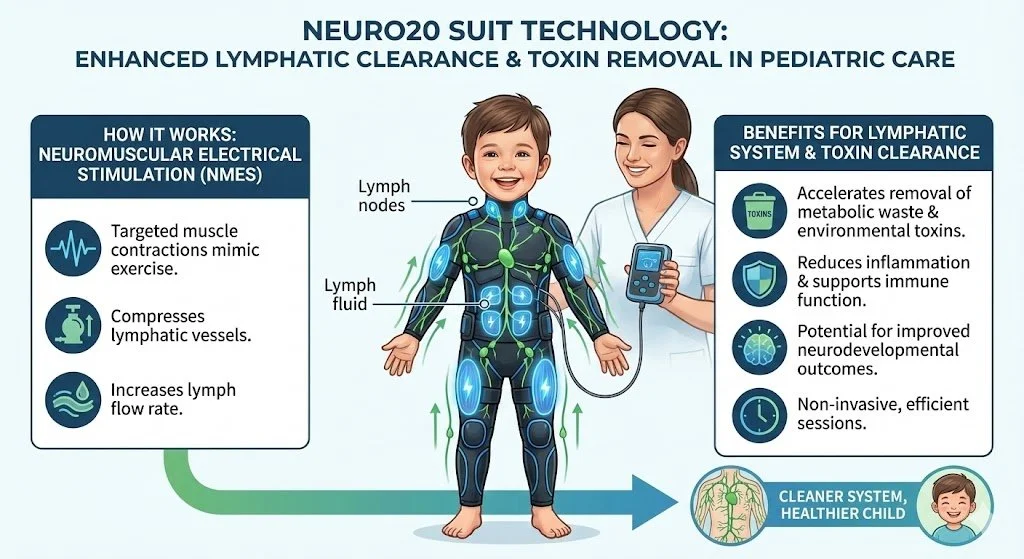
Neuromuscular Electrical Stimulation (Neuro20): The Systemic Flush
Mechanism: Contracts 20 muscle groups simultaneously. The Kynurenine Detox (The Muscle-Brain Rescue): Chronic inflammation converts the amino acid Tryptophan into Quinolinic Acid, a potent neurotoxin that causes agitation and anxiety. Skeletal muscle contraction (induced by Neuro20) produces enzymes that intercept this pathway, converting the chemicals into Kynurenic Acid, which is neuroprotective. Effectively, the muscles "filter" the blood of neurotoxins before they reach the brain [7].
Transcranial Magnetic Stimulation (TMS - Exomind): Rewiring the Loop
Mechanism: Uses magnetic fields to induce electrical currents in the Dorsolateral Prefrontal Cortex (DLPFC), helping the child override obsessive impulses [8]. Safety & Nuance: We utilize Low-Frequency (1Hz) inhibitory protocols for anxious patients to act as a "brake" on overactive circuits, ensuring safety and tolerability in pediatrics [10].
Biological Support: The Metabolic & Structural Foundation
With the biophysical "engine" restarted, specific biological co-factors become the limiting reagents. We utilize targeted molecules to fuel the repair process and resolve inflammation.
1. NAD+ (Nicotinamide Adenine Dinucleotide)
Justification: Critical fuel for mitochondrial ATP production and PARP enzymes (DNA repair).
Clinical Rationale: Reverses "cellular brownout" and fuels repair mechanisms.
2. Glutathione (and NAC)
Justification: The brain's master antioxidant.
Clinical Rationale: Modulates glutamate excitotoxicity and quenches oxidative fire.
3. Creatine Monohydrate
Justification: The brain's "backup battery" (phosphate donor).
Clinical Rationale: Improves cerebral bioenergetics and spares methylation resources.
4. Vitamin D
Justification: Potent immunomodulator for T-Regulatory cells.
Clinical Rationale: Stabilizes the autoimmune response and maintains barrier integrity [13].
5. Specialized Pro-Resolving Mediators (SPMs)
Justification: Active metabolites of Omega-3s responsible for the "Resolution Phase" of inflammation.
Clinical Rationale: PANS patients often lack the enzymes to convert fish oil into active Resolvins. SPMs act as the biochemical "off switch" for the cytokine storm, signaling the immune system to stop attacking and begin repairing [14].
6. Neuro-Flavonoids (Luteolin & Quercetin)
Justification: Potent stabilizers of Mast Cells and Microglia.
Clinical Rationale: Luteolin crosses the BBB. It synergizes with Green Light Therapy to inhibit mast cell degranulation (which keeps the BBB leaky) and calm activated microglia [15].
7. Zinc
Justification: Essential immune modulator and NMDA receptor regulator.
Clinical Rationale: Zinc creates a "calming" effect on the excitotoxic NMDA glutamate receptors involved in tics and anxiety.
Synthesis: The Integrated Clinical Cadence
A successful protocol must prioritize stabilizing the brain's physics. Patients typically undergo this integrated protocol 2-4 times per week, allowing for simultaneous:
Calming & Opening: PEMF & Green Light.
Flushing & Detox: Neuro20 (Kynurenine shunt & Lymphatics).
Energizing & Repair: tPBM & EWOT (Mitochondria & BBB).
Rewiring: TMS (Electrical homeostasis).
Fueling: NAD+, Glutathione, Creatine, Vitamin D, SPMs, Flavonoids, & Zinc.
A New Horizon for the Inflamed Brain
PANS/PANDAS is not a life sentence of psychiatric misery; it is a treatable medical condition rooted in neuroinflammation and biophysical failure.
The current reliance on biochemical manipulation is hitting a wall. We are trying to put out a forest fire with water pistols while the arsonist is still pouring gasoline. By integrating regenerative biohacking, we change the strategy. We fix the fence (BBB), we resupply the firefighters (neuronal energy), and we calm the winds (autonomic nervous system).
This "Physics-First" approach is not an alternative; it is a fundamentally sound approach for healing the "brain on fire." It offers a scientifically grounded path forward for the countless families navigating this devastating diagnosis.
Bibliography
Platt, M. P., et al. (2016). Th17 lymphocytes promote neuronal cell death in a model of pediatric autoimmune neuropsychiatric disorders. PLoS One.
Hornig, M. (2013). The role of microbes in the pathogenesis of neuropsychiatric disorders. Current Opinion in Rheumatology.
Salehpour, F., et al. (2018). Brain Photobiomodulation Therapy: a Narrative Review. Molecular Neurobiology.
Hamblin, M. R. (2019). Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned? Photonics.
Ibrahim, M. M., et al. (2021). Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons. Science Translational Medicine.
Pavlov, V. A., & Tracey, K. J. (2012). The cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity.
Agudelo, L. Z., et al. (2014). Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell.
Grados, M. A., et al. (2018). A review of TMS in pediatric neuropsychiatric disorders. Brain Stimulation.
Cassano, P., et al. (2016). Near-infrared transcranial radiation for major depressive disorder: proof of concept study. Psychiatry Journal.
Croarkin, P. E., et al. (2021). Transcranial magnetic stimulation in child and adolescent psychiatry: a systematic review and meta-analysis. Pediatrics.
Amen, D. G., et al. (2012). Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury. PLoS One.
Bloch, M. H., et al. (2016). N-acetylcysteine in the treatment of pediatric obsessive-compulsive disorder. JAACAP.
Mahdaviazad, H., et al. (2021). The status of Vitamin D in children with PANS/PANDAS. Journal of Child and Adolescent Psychopharmacology.
Serhan, C. N. (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature, 510(7503), 92-101.
Theoharides, T. C., et al. (2015). Brain "fog," inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Frontiers in Neuroscience, 9, 225.